This article was written by Chen Bing(Partner) and Yang Yue(Managing Associate).
Since the melamine milk scandal in 2008, there has been constant reform to the supervision of the infant formula industry. Registration of infant formula milk powder ("Infant Formula") is probably one of the most significant changes. It will greatly affect all the industry players.
From 1 January 2018, the Infant Formula registration requirements in the new Food Safety Law came fully into force. Infant Formula products, either domestically manufactured or imported through general trade, must obtain formula registration before they can be sold in the PRC. This requirement will impact thousands of Infant Formula brands in the market - a great portion of which will not survive this change.
It should be noted that Infant Formulas distributed through cross-border e-commerce retail[1] ("CBEC Retail") enjoy a grace period until the end of 2018 when another new policy will probably come into force. Details are set out below.
Overview of Legislative Progress
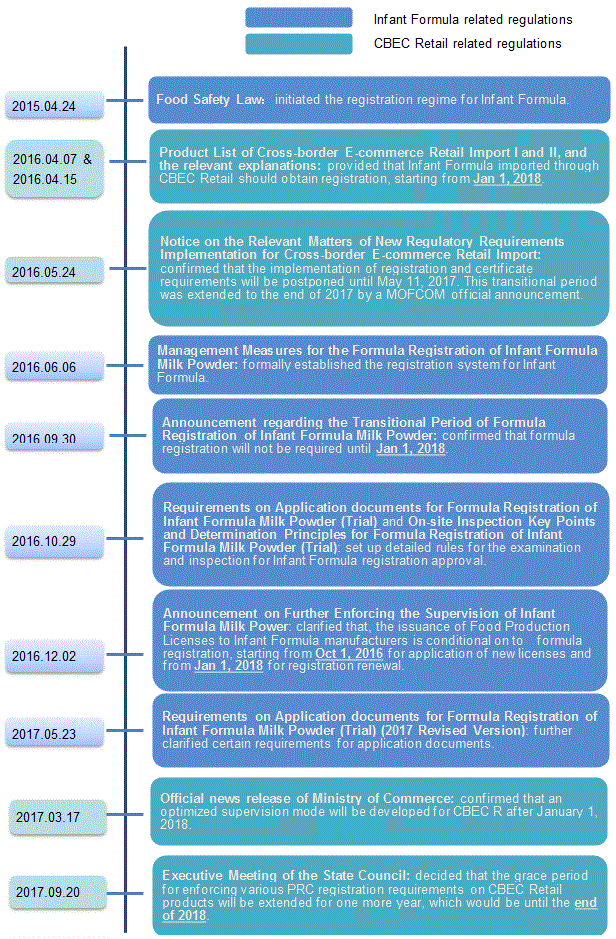
The supervision of Infant Formula has been developed under the Food Safety Law in April 2015 and has been perfected over the last (nearly) three years. A series of implementation rules are now in place, to establish a fully functional system for supervision of Infant Formula.
Infant formula distributed through CBEC Retail will eventually be subject to registration requirements. Although, CBEC Retail rules for infant formula have evolved in a different way from the rules that apply to general trade.
Below is a timeline of relevant regulations and circulars relating to infant formula registration requirements:
Formula Registration Requirements
Key points on the current law regarding Infant Formula registration are:
All Infant Formula products to be sold in China, whether domestically manufactured or imported, must obtain formula registration from China Food and Drug Administration ("CFDA");
The maximum number of formulas that can be registered by a company is nine (9), and such formulas shall be allocated to no more than 3 product series ("Formula Quota");
A wholly owned subsidiary can use formula registered by an affiliate of the same group company provided that (i) the subsidiary has obtained its own formula registration and manufacturers license before using the formulas owned by the affiliate, (ii) the subsidiary has the necessary technology, equipment and facilities for manufacturing, and (iii) the group company files a written report with CFDA beforehand.[2]
Current Status and findings
By 11 January 2018, there were 1000 formulas registered with CFDA by 134 companies. Among them, 96 were domestic manufacturers (including foreign invested companies) and 38 were foreign manufacturers[3].
Our analysis of the data and information in the formula registration lists, revealed the following:
1. Formula Quota limitation applies to a single entity (not a group).
The relevant Infant Formula registration rules do not state whether the Formula Quota limitation (3 series, in total of 9) applies to an individual entity or to a group of companies. In other words, it is not clear whether each subsidiary of a group company is entitled to apply for the Formula Quota independently.
In the formula registration lists issued by CFDA, there are examples where affiliates of a group company have successfully accumulated more than 9 formulas. Therefore, it can be concluded that each of the affiliated companies can enjoy an independent Formula Quota, as long as the application thresholds are met.
2. "All in" or "save for later"
Among the 134 manufacturers, 87 had registered 9 formulas. So, more than half of the applicants had consumed their entire Formula Quota. But a fair number of manufacturers had saved some of their Formula Quota for later. Among those who had consumed their entire Formula Quota, only 37 were from a foreign investment background (foreign company/foreign invested company). The remaining 50 were pure domestic enterprises. The companies with 9 registered formulas were mostly well known brands.
3. Domestic brands vs foreign brands
Foreign companies account for only 28% of the companies with infant formula registrations. But, this does not necessarily mean international brands have lost their dominance in the sector. Of the 134 manufacturers who have registered Infant Formulas, about 40% of them are pure domestic companies while about 60% have a foreign investment element[4].
4. On-site inspections of Foreign Manufacturers - not strictly implemented
The Management Measures for Formula Registration of Infant Formula Milk Powder allow the CFDA to conduct on-site inspections of applicants, including foreign applicants, at the CFDA's discretion. However, the procedure for foreign inspections is unclear.
According to anonymous consultations with the CFDA, so far no foreign on-site inspections have been conducted. This is not surprising given the tight schedule by which CFDA have to handle tons of applications from market players. However, how the CFDA will conduct the on-site inspections (if at all) remains unclear.
Can's and Cannot's after 1 January 2018
Cannot
Infant formula products cannot be distributed or sold in China without formula registration. This applies whether the products are manufactured in China or imported to China through general trade. Although the following exceptions apply:
Cans (Exceptions)
(1) Transition arrangement – according to the updated rule[5] (November 2017) , Infant Formula manufactured by foreign manufacturers before 1 January 2018 can be imported and sold before its expiration date without formula registration.
(2) Survival of CBEC model
Compared with the steadily evolving Infant Formula supervision system, the development of CBEC Retail over the past two years is like riding a roller coaster! Along with the initiation of formula registration requirements, there has been some back and forth on whether (and when) the registration requirements should also apply to CBEC Retail.
According to the latest policy announced in September 2017 following the Executive Meeting of the State Council, the grace period for enforcing various PRC registration requirements on CBEC Retail products will be extended again for one more year, i.e. until the end of 2018. That means that Infant Formula products can still be distributed in China until the end of 2018 through the CBEC Retail model without a registration. This will offer a breath-catching period to those foreign brands which have not yet obtained registrations.
However, despite the constantly changing policies of CBEC Retail, another extension of the application period is unlikely. Considering that an application for an Infant Formula registration requires a fair amount of preparation, it is suggested that foreign Infant Formula brand owners who are relying on the CBEC Retail model should prepare to apply for registration as soon as possible.
[1]Meaning sales where the products are sold through an e-commerce platform connected to PRC Customs, and the products will be delivered to PRC consumers either directly from overseas or from a PRC bonded zone.
[2]For details, see Notice on Matters related to the Use of Registered Formula of Infant Formula Milk Powder of the Wholly Owned Subsidiaries of a Group Company, issued by CFDA on December 13, 2017.
[3]According to announcements made on CFDA's official website
[4]Based on our check of shareholding registration information of the concerned companies disclosed on National Enterprise Credit Information Publicity System.
[5]Announcement on the Implementation Date of Formula Registration for Import Infant Formula Milk Powder, issued by CFDA and General Administration of Quality Supervision, Inspection and Quarantine on November 10, 2017.